“There is no formula for success, except perhaps an unconditional acceptance of life and what it brings,” Hotchner quoting Arthur Rubinstein.
Criminal Minds, Season 3, Episode 15, “A Higher Power”
This is Sara here and this week we’ll discuss the topic of Nucleic acids and nucleotides. So what are nucleotides and why do we care? Well, we cannot exist without nucleotides!They are the elementary structures used to make nucleic acids, such as DNA and RNA.

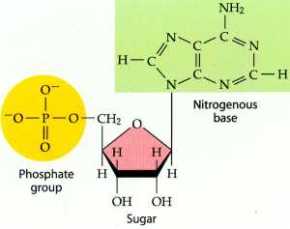
A nucleotide is an organic compound made up of a phosphate group, nitrogenous base and a sugar, and it contains the following elements of carbon, nitrogen, oxygen and phosphorous.
Now in order to understand nucleic acids and nucleotides, we need to familiarize ourselves with some new terms or may be not so new. The first is Pyrimidines, which are aromatic heterocyclic organic compounds similar to pyridine, (a simple heterocyclic organic compound (C5H5N). They occur in DNA as cytosine and thymine, which are the pyrimidines in RNA.
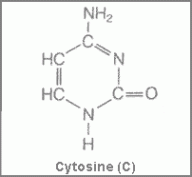
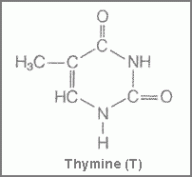 Figure 1 showing structure of cytosine Figure 2 showing the structure of thymine
Figure 1 showing structure of cytosine Figure 2 showing the structure of thymine
Next, are Purines which are heterocyclic aromatic organic compounds which occur as adenine and guanine in DNA and RNA.
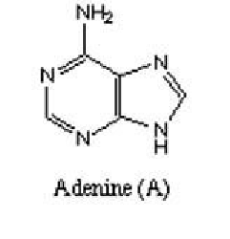
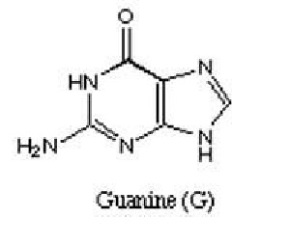
Figure 3 showing structure of adenine Figure 4 showing structure of guanine
So, Nucleotides are necessary to us as most importantly, they are used as an energy currency in cellular respiration, in the form of ATP, as allosteric effectors (see enzymes) as well as the structural components of enzyme co-factors. Nucleotides are the phosphoric esters of nucleosides.
Then we also have Nucleosides which are pyrimidine or purine N-glycosides of D-ribofuranose. The purine or pyrimidine part of a nucleoside is referred to as a purine or pyrimidine base. The main component of a nucleoside is the phosphate group as it is necessary for nucleotide polymerization.
Pyrimidine and purine nucleosides of D-ribofuranose include Uridine and Adenosine. Uridine contains a uracil group linked to a ribose ring by a Beta-N1 glycosidic bond while Adenosine has a molecule of adenine bonded to a ribose ring by a beta-N9 glycosidic linkage.
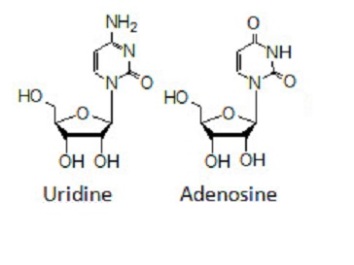
Figure 6 showing Uridine and Adenosine structures

Nucleotides form Nucleic acids which include DNA and RNA.DNA has two strands, is a deoxyribose sugar and is characterized by 4 bases; Guanine (G), Cytosine (C), Adenine (A) and Thymine (T).
RNA also has four bases, G, A, C and instead of T, there is Uracil (U). It possesses a single strand and can have the form of rRNA (RNA + Protein), mRNA, and tRNA.

OK, well, rRNA are ribosomal RNAs which function in protein synthesis, mRNA are messenger RNAs which transport genetic information from genes to ribosomes. tRNA are transfer RNAs which translate information from mRNA to amino acid sequences.
Nucleic acids are formed by nucleotide monomers forming phosphodiester linkages between the 3’-OH of one nucleotide and the phosphate of another nucleotide.They can have 3 different forms: A, B and Z. A is common for RNA and DNA, B is favoured by RNA and Z does not occur generally but can form for some DNA sequences.
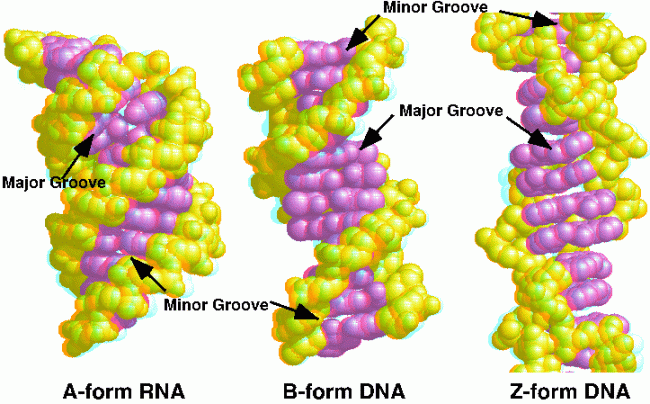
Figure 7 showing A, B and Z forms of DNA and RNA
The major molecule which encodes genes containing information for the formation and function of most if not all living organisms is…yes you know it, DNA! The ‘backbone’ of DNA (deoxyribose sugar) comprises of five carbons which are numbered as 11, 21, 3 1, 4 1and 5 1 and three oxygen. Phosphate groups are linked by the hydroxyl groups present at carbons 31and 5 1. The A, G, C and T bases can extend from the chain in order to stack on top each other which contributes to the stability of the nuclei acid as this is more energetically favourable.
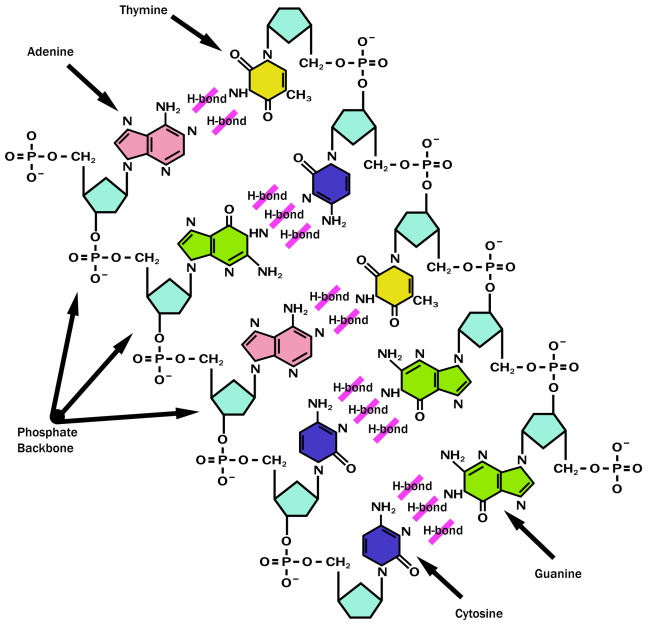
Figure 8 showing the structure of DNA
Base pairs are similar in size and shape which allows for more efficient packing into the double helix. Bases are linked by hydrogen bonding and are hydrophobic and therefore located to the inside of the helix, perpendicularly stacked and the phosphate groups are polar so they are located to the outside. This is described as being hypochromic which also makes bases less susceptible to UV absorption.
DNA molecules are generally circular in shape in eukaryotes and are usually negatively coiled as this form has a higher torsional energy allowing for unwinding of the helix during transcription or replication. Having supercoiling in the DNA tertiary structure reduced the problem of the lack of space, as the number of base pairs and length per 360 degree turn was equivalent to one meter!

Things seem to be going well for DNA and RNA but acids, alkalis and other chemicals can have negative effects on the structure of DNA and RNA. Denaturation can also occur due to chemicals such as urea and formamide which disrupt H bonding and hydrophobic effects and thermally by high temperatures which destroy double stranded H bonded regions. However renaturation can occur by rapid cooling, annealing (base pairing of short regions complementarily) and hybridization.

Well, that’s all for now!

























 Figure 1 showing structure of cytosine Figure 2 showing the structure of thymine
Figure 1 showing structure of cytosine Figure 2 showing the structure of thymine